Title: NASH Cirrhosis Space Poised to Heat Up
Author: Peter G. Traber, MD
NASH cirrhosis is the most advanced classification of fibrosis progression in fatty liver disease. Results of clinical trials in this space have been rather sparse and either negative or of moderate efficacy, but clinical trial data is about to take a major upswing. On September 25, 2018, I spoke at Discovery on Target’s 2nd Annual NASH and Fibrosis conference on “Therapeutic approaches to cirrhotic versus pre- cirrhotic NASH”, where I highlighted the pathophysiology of NASH cirrhosis and reviewed the clinical trials currently underway with expected timelines for top-line results.
View my full presentation:Therapeutic approaches to cirrhotic versus pre-cirrhotic NASH
Reported NASH Cirrhosis Clinical Trial Data
To date, two drugs have shown potential for therapy of NASH cirrhosis in phase 2 studies. Galectin Therapeutics (NASDAQ: GALT) reported Phase 2b clinical trial results which showed that GR-MD-02 reduced portal pressure (HVPG) and prevented the development of new varices in patients with compensated cirrhosis and no varices at baseline, in a post-hoc analysis of that patient subset. Conatus Pharmaceuticals (NASDAQ: CNAT) showed in advanced stage cirrhotic patients that emricasan was able to reduce portal pressure and improve laboratory indices of liver disease severity. Both company’s results require additional prospective clinical data targeting the specific patient populations to confirm the effect seen from patient subset analysis in the original studies. As shown below, there is additional data to soon be reported by Conatus for emricasan in those patient groups, and Galectin is planning for a phase 3 clinical trial in the same population were positive results were seen in phase 2.
NASH cirrhosis has been a challenging therapeutic space, as demonstrated by the failure of Gilead’s simtuzumab. This monoclonal antibody inhibitor of LOX-L2 is a promising putative antifibrotic, but it had no effect in NASH cirrhosis nor fibrosis in pre-cirrhotic NASH. Gilead dropped the program after a robust phase 2 clinical trial effort.
NASH Cirrhosis Clinical Trials and Milestones
As shown in the chart below, there are now five drugs in development for NASH cirrhosis, with seven ongoing clinical trials, including two phase 3 trials, one phase3 ready trial, and 4 large phase 2 trials. With four of the drugs, these NASH cirrhosis trials are supported by clinical trials in pre-cirrhotic NASH, including two phase 3 and two phase 2 trials, which are also designed to demonstrate an anti-fibrotic effect.
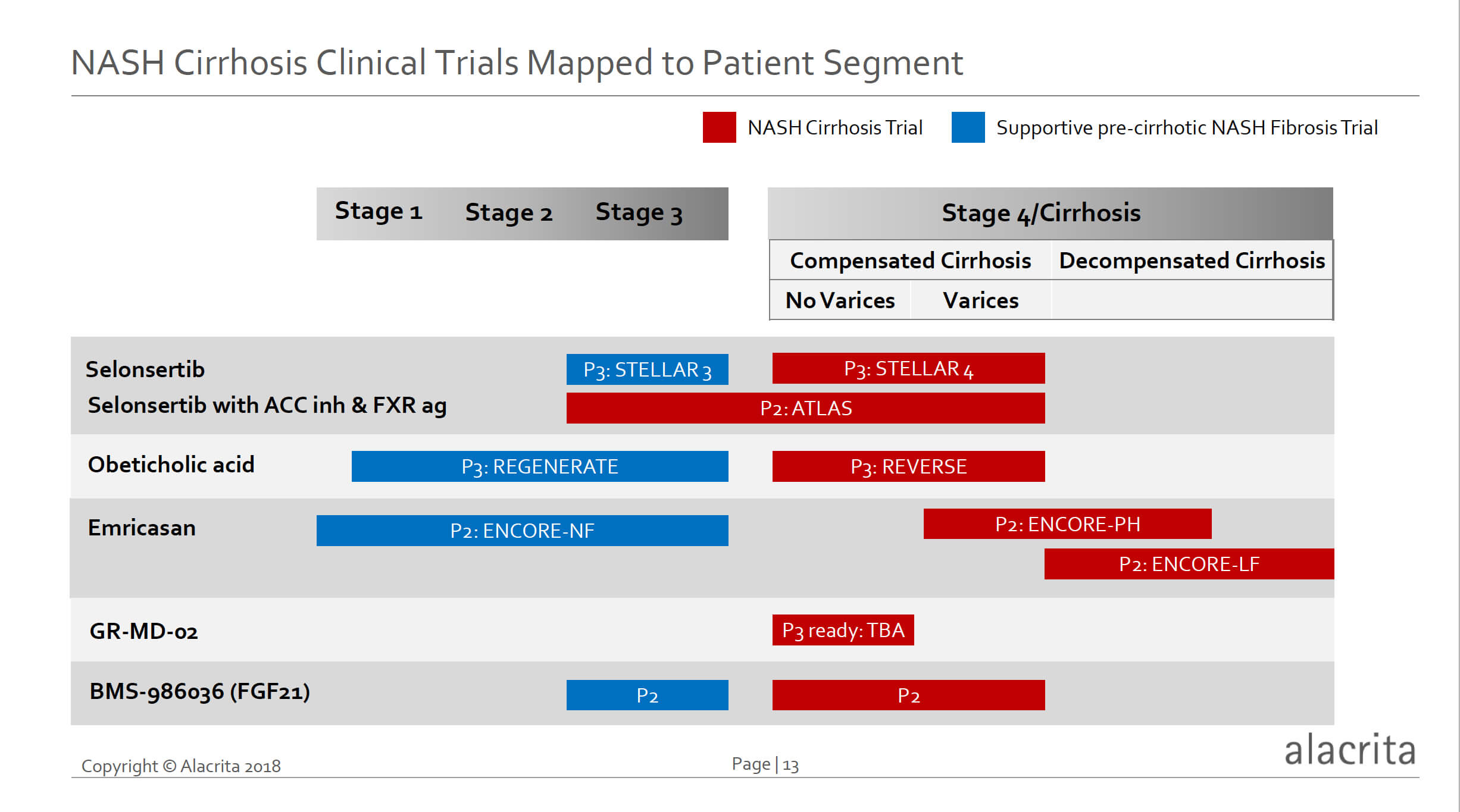
Many of these studies are well advanced, and as shown in the figure below there are multiple relatively near-term data readout milestones. Gilead’s phase 3 STELLAR-3 and 4 trials will both have data in the first half of 2019. If the STELLAR-4 trial hits its endpoint of a one stage reduction of fibrosis (essentially reversal of cirrhosis), this would likely herald the first approved NASH cirrhosis drug. STELLAR-3 will provide an opportunity for drug registration in pre-cirrhotic NASH as well.
Conatus Pharmaceuticals, and its partner Novartis, will soon have data from two phase 2 clinical trials in advanced cirrhosis, including ENCORE-PH in Q4 2018 where the primary endpoint is change in portal pressure (HVPG), and ENCORE-LF in the first half of 2019 where the primary endpoint is improvement in time to first clinical decompensation event. These two trials, as mentioned above, will served to validate findings from smaller phase 2 studies previously reported.
Intercept Pharmaceuticals phase 3 REGENERATE trial in pre-cirrhotic NASH will provide insight into the likelihood of success with the REVERSE cirrhosis trial to read out in mid-2020. BMS’s FGF-21 analog is scheduled to readout data on both NASH cirrhosis and pre-cirrhotic NASH in Q1 2020. Galectin’s phase 3 trial design and timings have not yet been announced.
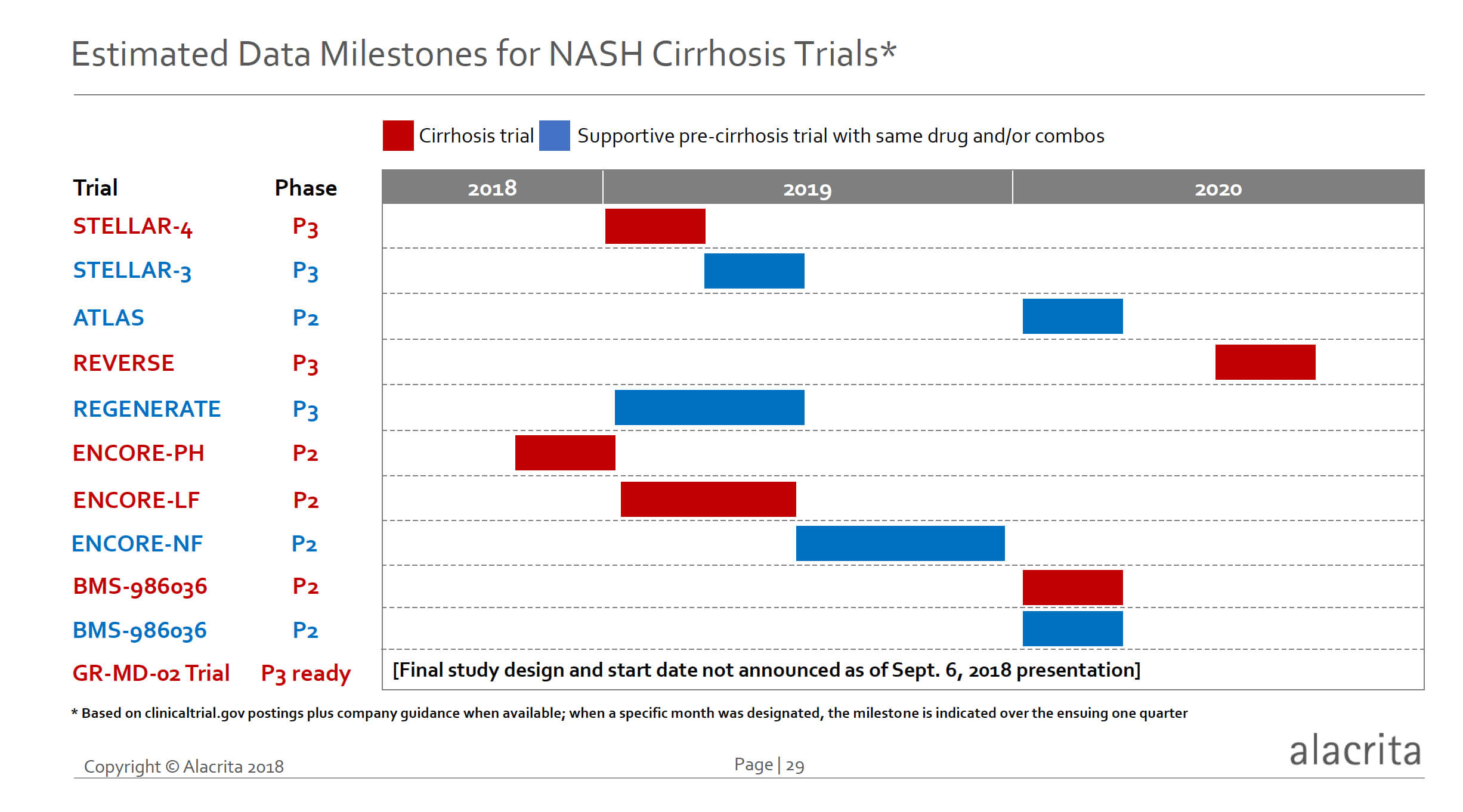
The next 18 months will provide an abundance of data on various potential therapies for NASH cirrhosis. The promise of new therapies as well as natural history and clinical data on NASH cirrhosis will make this an exciting period in NASH drug development. As a disclaimer, I have not reviewed any drugs in development for pre-cirrhotic NASH unless they also have a concomitant cirrhosis trial ongoing. The large amount of expected data from other pre-cirrhotic NASH trials will additionally inform potential therapies for NASH cirrhosis, including rational combinations.
To read and receive other insights and commentary on drug development, please subscribe above or via my page here.
Disclosure: From March 2011 through June 2018 the author was CEO and CMO of Galectin Therapeutics (NASDAQ: GALT), a company with a NASH development asset, and continues to hold equity in the company.

