Many would agree that input assumptions are the most important part of a pharma or biotech portfolio valuation. Of these, one particularly critical assumption is the addressable patient population, which is dependent on the epidemiology of a specific disease. Whilst finding epidemiological figures for well-researched diseases is often straightforward, the task becomes particularly complex when you consider poorly reported incidence and prevalence rates for rare and ultra-rare indications.
In the context of disease, prevalence is defined as ‘a measure of the burden of disease in a population in a given location and at a particular time, as represented in a count of the number of people affected’ (Ward, 2013). This definition is equivalent to the equation below:
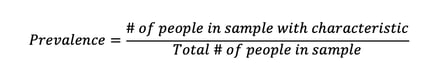
Incidence, on the other hand, represents the number of new cases in a certain time period, expressed as a risk or an incidence rate. The important distinction to make, therefore, between incidence and prevalence is the consideration of time – prevalence represents the current or past state of a population, whilst incidence allows for the prediction of future events within a population.
The prevalence of a condition is dependent on the disease incidence, the deaths and the recoveries, as outlined in Figure 1. For example, if we assume the population is at steady state i.e. the prevalence is fairly constant and cure and deaths are equal, the prevalence is approximately equal to the product of incidence rate and mean duration of the disease.

Figure 1: The relationship between incidence and prevalence
Typically, the prevalent population is greater than the number of incident cases; this is because in each year, a relatively small number of individuals are diagnosed with a certain disease (incidence) in comparison with the total number of patients with the same disease within a population (prevalence) at a given point in time. For biotech and pharma valuations, it is important to model the most appropriate target patient population using either the prevalent population or incident cases. Whether to incorporate the prevalent population or incident case when modelling the value of a drug depends on the nature of the treatment itself e.g. a one-shot cure vs a daily administered drug patients must take life-long. For example, when administering a one-time gene therapy to treat an incurable disease, the target patient population would initially be the prevalent population which would result in a bolus of treatments in the first few years of the gene therapy post-launch, followed by incident cases year on year going forward. On the other hand, for a drug like Prozac for management of depression, it would be most appropriate to model the prevalent population year on year.
Whilst the concept of incidence and prevalence is well and good, both have their limitations. For example, incidence is more useful than prevalence in understanding disease aetiology; this is primarily because prevalence is scaled by the average life expectancy of a disease, whilst incidence is not. Furthermore, the incidence and prevalence of a population are highly dependent on the criteria used to diagnose a disease; populations with stricter diagnostic criteria are likely to be lower than those with lax criteria. For these reasons, it is crucial that we carefully consider the appropriateness of incidence and prevalence in biotech and pharma valuations.
Bibliography
Ward, M. M., 2013. Estimating Disease Prevalence and Incidence using Administrative Data: Some Assembly Required. The Journal of Rheumatology, 40(8), pp. 1241-1243.
Authors
Saadia Basharat, PhD, Senior Consultant and Qassi Gaba, Consultant Intern

