The purpose of this analysis of patient-reported outcomes from the ELECT (Evaluation of Lanreotide Depot/Autogel Efficacy and Safety as a Carcinoid Syndrome Treatment) trial (NCT00774930) was to explore the effect of lanreotide on symptoms of carcinoid syndrome. Specifically, this post hoc analysis was designed to identify the most important patient-reported outcomes for patients in ELECT.
Methods: The post hoc analysis of ELECT, a placebo-controlled study of lanreotide in patients with neuroendocrine tumors, evaluated patient-reported outcomes during the doubleblind phase of the trial, specifically daily diarrhea and flushing symptoms, octreotide rescue use, and the EORTC QLQ-C30 and QLQ-GINET21 questionnaires at baseline and week 12. Principal component (PC) analysis was applied on baseline data to identify independent variable clusters and clinically meaningful summary measures that highly correlated to these PCs. From those, the minimum clinical important differences were derived so to perform a responder analysis.
Results: The three largest PCs captured 42.9% of the variation among baseline variables. The C30 summary score (C30-SS), diarrhea burden, and flushing burden were highly correlated with PC1, PC2, and PC3, respectively. Lanreotide patients were more likely to experience an improvement on the C30-SS (risk ratio [RR] 2.42; P=0.023), diarrhea burden (RR 2.85; P=0.005), and flushing burden (RR 1.39; P=0.31) compared to placebo patients. Lanreotide-treated patients have a higher probability of being a responder on at least one of the three domains of C30-SS, diarrhea burden, or flushing burden compared to placebo patients (RR 1.48; P=0.06).
Conclusion: The higher response rates in the diarrhea burden are consistent with the previously reported effects of lanreotide on octreotide rescue medication use, while the findings of a greater efficacy of lanreotide vs placebo in the quality-of-life domains represent a novel aspect in the benefits of lanreotide.
Trial registration: ClinicalTrials.gov identifier: NCT00774930.
Keywords: lanreotide, patient-reported outcomes, neuroendocrine tumors, NETs
Introduction
Neuroendocrine tumors (NETs) originate from pancreatic islet neuroendocrine cells, diffuse gastroenteric neuroendocrine cells, and/or neuroendocrine cells elsewhere in the body.1,2 The diffuse neuroendocrine system cells are scattered throughout organs such as the esophagus, stomach, pancreas, small and large intestines, and lungs. Tumors arising from the diffuse neuroendocrine system have been known for more than a century as carcinoid, midgut, or gastrointestinal (GI) tumors.
NET is considered a rare form of cancer and is most often of indolent nature, histopathologically well differentiated,1 with a subset of patients who experience symptomatic disease due to the production of bioactive amines.3 Symptoms can be secondary to local tumor mass effects or, most often, as a result of hepatic metastases and evoked by the release of hormones (eg, serotonin) directly into the systemic circulation.4 The ability to secrete bioactive amines and the type of secretions varies widely across neuroendocrine primary tumor types, with tumors arising from the midgut (colon and small intestine) being most likely to lead to carcinoid syndrome (CS).2 Serotonininduced CS often manifests as episodes of flushing and diarrhea5,6 and, in chronic cases, as right-sided valvular heart disease.7
In many diseases, such as metastatic cancer, the goal of therapy is to alleviate symptoms, increase daily functioning, and/or improve quality of life (QoL). Data reported from patients are needed to understand the full impact of disease in addition to clinical and laboratory assessments.8 Thus, to capture the impact of an intervention on subjective concepts such as symptom burden or functional aspects, the US Food and Drug Administration (FDA) recommends utilizing psychometrically validated patientreported outcome measures (PROMs) in clinical trials.9 Due to the symptomatic nature of NETs and the disease impact on QoL, PROMs have become common in many prospective, interventional clinical trials.10–13
Lanreotide has been recently shown to reduce the need for subcutaneous (sc) octreotide for symptom control in patients with CS through a Phase III, randomized, controlled, double-blind trial (Evaluation of Lanreotide Depot/ Autogel Efficacy and Safety as a Carcinoid Syndrome Treatment [ELECT]).13 However, due to the heterogeneity of the clinical manifestations of CS, it was anticipated that no single measure would be able to broadly capture treatment benefit.
To comprehensively assess the effect of lanreotide treatment on patients’ daily functioning, this post hoc exploratory analysis defined “carcinoid syndrome response” using the set of patient-reported outcomes from the ELECT study. Specifically, this post hoc analysis further characterizes the effects of lanreotide on the control of symptoms associated with CS, with a focus on the clinical meaningfulness of the patient-reported outcomes.
Principal component analysis (PCA) methodology was used as an operator-independent method to select the most relevant patient-reported outcomes. Although principal components are often challenging to understand clinically, it may be helpful to replace the abstract principal component by a highly correlated variable (“response measure”) that is easier to understand from a clinical point of view. To characterize potential CS response measures, correlations between each of three principal components (PCs) and the PCA variables, as well as potentially suitable composite score, were explored. For the primarily QoL-driven PC1, the C30 summary score (C30-SS), which was previously described and validated by Giesinger et al (2016),14 was the most highly correlated variable (r=0.943) and was therefore selected as a clinical proxy for PC1 (Supplementary Annex 1, Table S1). This summary score combines all functional and symptom subscales except for the QL (global quality of life) and FI (financial difficulties) subscales.
It was observed that for diarrhea and flushing, respectively, symptom frequency and severity contributed equally to the loading of the respective PCs. Therefore, diarrhea and flushing composite “burden” variables were calculated by averaging baseline symptom frequency and severity. Burden of diarrhea (BD) showed the highest correlation with PC2 (r=0.854), while burden of flushing (BF) showed the highest correlation with PC3 (r=0.725) (Supplementary Annex 1, Table S1).
The clinical benefit to the patient, referred to as “CS response,” is characterized as the functional benefit experienced by the patient either in terms of an improvement in symptom endpoints or in day-to-day functioning and QoL endpoints. A further analysis conducted as part of this post hoc investigation was the association between the primary ELECT endpoint (ie, use of sc octreotide rescue medication) and a patient-reported CS response.
Methods
ELECT Phase III Clinical Trial
The ELECT study protocol and related materials were reviewed and approved by an Independent Ethics Committee/Institutional Review Board prior to commencement of the study in all countries where the study was conducted (refer to Supplementary Annex 2). The study was conducted under the provisions of the Declaration of Helsinki, and in accordance with the International Conference on Harmonisation Consolidated Guideline on Good Clinical Practice. Informed consent was obtained before initiation of any study-related procedure and administration of the study treatment. ELECT (ClinicalTrials.gov identifier: NCT00774930) was a Phase III, multicenter, double-blind (DB), placebo-controlled randomized trial designed to evaluate the efficacy of the long-acting formulation of lanreotide 120 mg, compared with placebo, administered every 4 weeks for the control of symptoms associated with CS, through reduction in use of short-acting sc octreotide.13 In brief, adult patients (N=115) with a confirmed NET and CS diagnosis who were either treatment-naïve or whose CS symptoms were responsive to conventional doses of octreotide LAR or sc octreotide, were randomized and enrolled (1:1) to lanreotide (n=59) or placebo (n=56) to participate in the trial. The study consisted of a 28-day screening period (where patient-based data on CS symptoms were collected) followed by a 16-week double-blind phase in which patients were able to cross over to an open-label extension phase after 28 days if sc octreotide was taken for ≥21 days of the 28-day cycle and at a dose ≥300 μg/day for ≥14 of the 21 days, regardless of presence/absence of symptoms.
A multidimensional set of patient-reported outcomes were evaluated, including CS symptoms, QoL, and rescue medication endpoints, throughout the screening and DB phases of the ELECT trial. Patients were required to report the incidence and severity of the two most common and bothersome CS symptoms (diarrhea and flushing5,6) on a daily basis using an interactive voice response system, beginning at screening (4 weeks prior to baseline) and through the DB phase of the study (baseline through 16 weeks). Patients reported if either diarrhea or flushing was present, the number of events on a daily basis, and the overall event severity on a 3-point Likert scale (1 = mild, 2 = moderate, 3 = severe). The European Organisation for Research and Treatment of Cancer core Quality of Life Questionnaire module (EORTC QLQ-C30, version 3.0) and 21-item disease-specific QoL for gastrointestinal NETs (QLQ-GINET21) questionnaire were administered at baseline and at week 12 of the DB phase. The QLQ-C30 is a multidimensional QoL questionnaire composed of six multi-item functional scales (physical, role, cognitive, emotional, social, three multi-item symptom scales [fatigue, nausea and vomiting, and pain]), six single-item symptom scales (dyspnea, insomnia, appetite loss, constipation, diarrhea, financial impact), and a two-item global quality-of-life scale (QL). The analysis focuses on the QoL C30-SS only, rather than on the total 15 outcomes separately, as recommended by the EORTC Quality of Life Group. The QLQ-C30 summary score was found to exhibit equal or superior known-groups validity and responsiveness to change over time as compared to the individual QLQ-C30 scales.14
Responder Analysis Through PCA
To assess CS response, the exploratory analysis consisted of the following phases: (1) defining the CS response measures (through data reduction techniques); (2) defining response categories for each CS response measure by deriving minimal clinically important differences (MCIDs); (3) assessment of treatment efficacy (responder analysis); and (4) assessment of the association between the CS response measures and the ELECT primary endpoint.
Principal component analysis was used to reduce the dimensions of the available baseline patient-reported data. Available data for analysis included the frequency and severity of flushing and diarrhea, as well as the EORTC QLQ-C30 and QLQ-GINET21 subscale. The average percentage of days of sc octreotide use during screening was included as a proxy for symptom severity. Correlation coefficients were generated to identify clinical proxies for the identified PCs. In order to define patients as responders based on the identified PCs, the MCID was defined via a distribution-based method, the standard error of measure, for the QoL score or through an anchor-based method, the evaluation of receiver operator characteristic (ROC) curves with the goal of maximizing true positives and true negatives vs external anchor variables, for the symptom-based scores. Additionally, the entire distribution of responders for treatment and control was explored through cumulative distribution of response curves.9 Supplementary Annex 3 details recommended methods and references for determining responsiveness and minimal important differences for patient-reported outcomes (PROs), as well as the approach followed in this analysis.
Having defined the MCID for each CS response measure, patients were identified as responders for a given measure if the improvement from baseline to week 12 exceeded the measure’s MCID. Patients were considered non-responders if they showed symptom worsening, if symptom improvement was smaller than the MCID, or if they discontinued treatment or crossed over to the openlabel extension during the DB phase prior to week 12. A composite CS response measure was generated, being defined as the number of patients with a clinically meaningful improvement on at least one of the individual CS response measures. RRs comparing the lanreotide group with placebo were calculated for all binary NET response measures using exact methods and were tested by a chisquare test at the 5% significance level.
To assess the relationship between the ELECT primary endpoint (percentage of days with rescue medication in the DB period) and CS response, the association between the percentage of days with rescue medication use in the CS response measures was calculated using a logistical regression model with the binary NET response measures as response variables and the percentage of days with rescue medication in the DB period (the primary endpoint) as continuous fixed effect variable. The odds ratio (OR) was utilized as a summary statistic and a chi-square test at the 5% significance level was used to test for statistical significance. For further information, see Supplementary Annex 1.
Results
Responder Analysis Through PCA
The three first PCs captured 24.5%, 10.3%, and 8.1%, respectively, of the overall variation in the dataset, and together accounted for 42.9% of the overall variation in baseline patient-reported outcomes. PC1 was highly (r=0.94) correlated with the EORTC QLQ C30-SS, with an MCID of 5.65. PC2 and PC3 correlated best with measures that averaged the frequency and severity of diarrhea and flushing. These measures, classified as BD and BF, had MCIDs of –0.62 and –0.31, respectively, with negative changes from baseline exceeding the MCID, indicating clinically meaningful improvement. Details on the composition of the PCs and the derivation of MCIDs are available in Supplementary Annex 1 and Supplementary Annex 3, respectively.
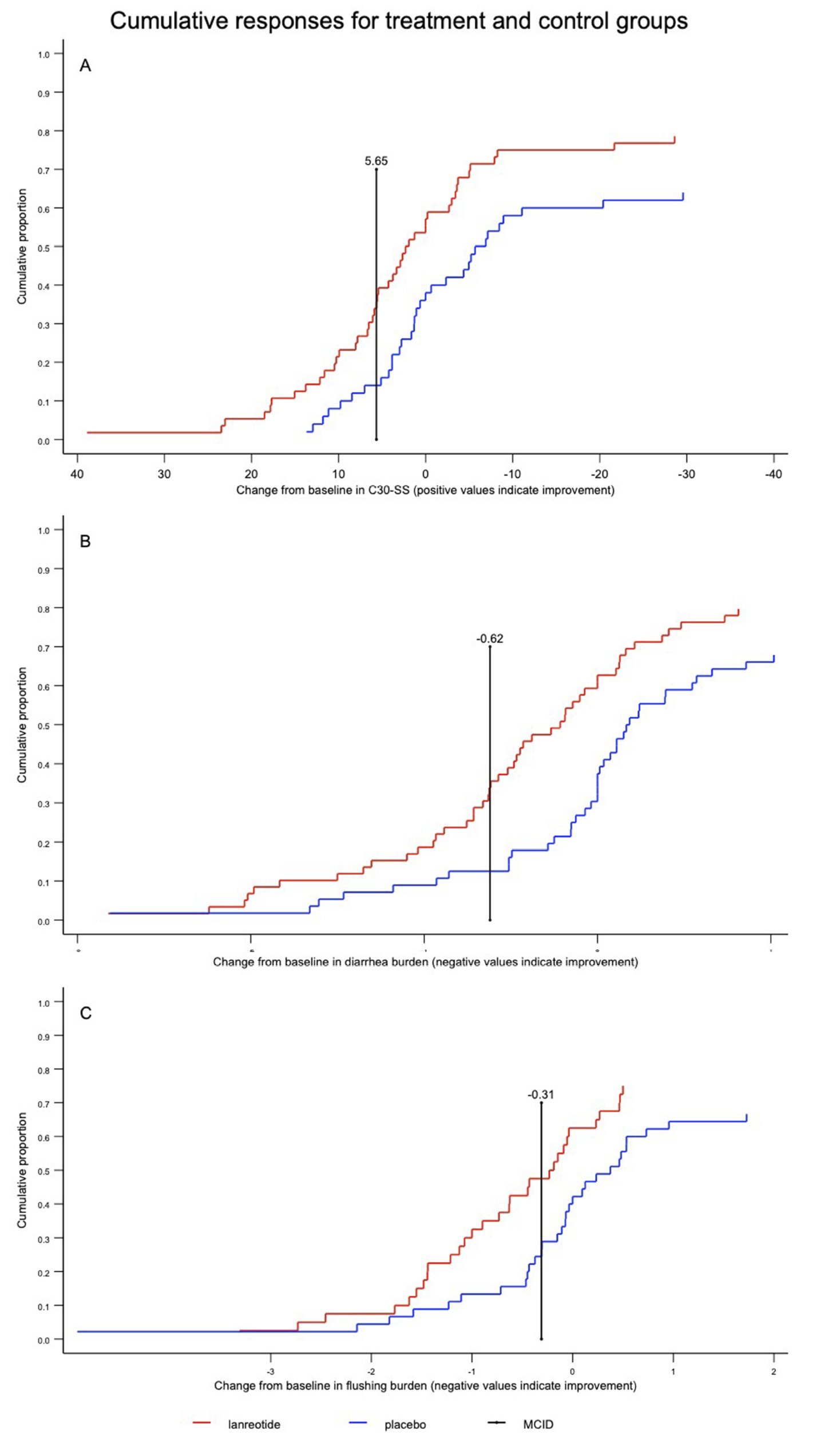
The results of the responder analysis are summarized in Table 1. The lanreotide group had a higher response rate for the C30-SS (RR=2.42; 95% CI=1.11–5.28) as well as for BD (RR=2.85; 95% CI=1.31–6.17). For the BD endpoint, 36% (21/59) of lanreotide-assigned patients were responders, compared to 13% (7/56) for the placeboassigned group (absolute risk difference = 23%; 95% CI=8–38%). BF response rates were numerically higher for lanreotide (RR=1.39; 95% CI=0.76–2.54). Among lanreotide- assigned patients, 58% (34/59) were composite responders to at least one of C30-SS, BD, or BF, as compared to 39% (21/54) of placebo patients (RR=1.48; 95% CI=0.99–2.21).
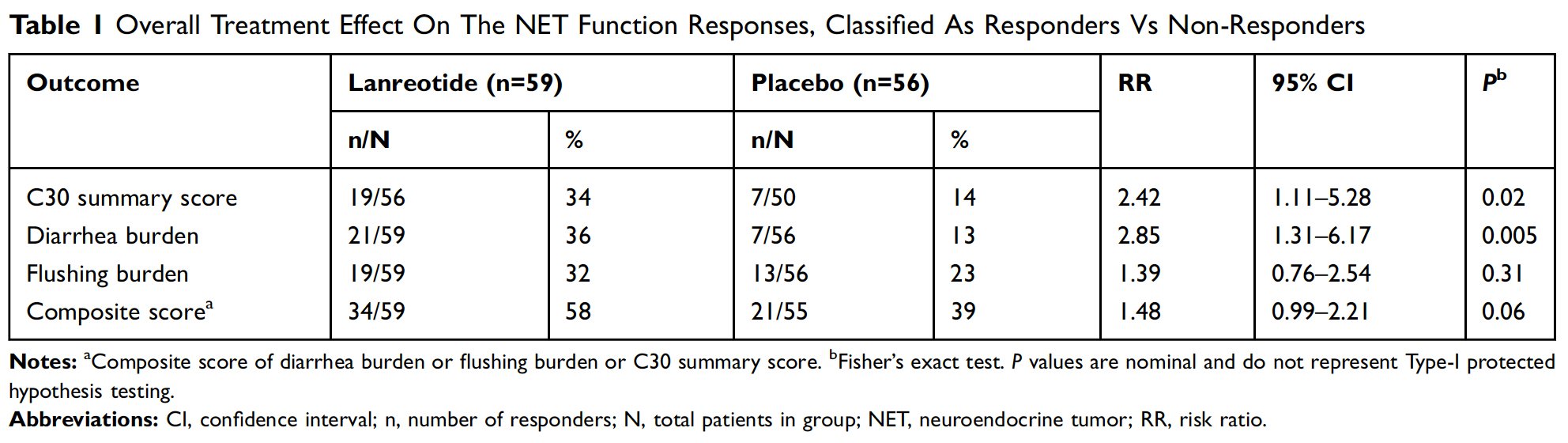
Figure 1A–C are graphical representations of response to treatment through cumulative distribution function curves for C30-SS, BD, and BF, respectively, from baseline to week 12. As demonstrated by the curves, the response to lanreotide was generally greater across the continuum regardless of the position of the MCID.
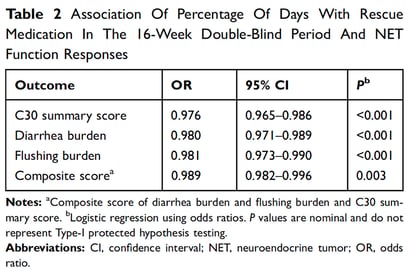
The results of the logistic regression examining the association between sc octreotide use during the DB phase and the CS function response measures are summarized in Table 2. For the BD and C30-SS responses, the odds of a response decreased with increasing percentage of days of rescue medication use in the 16-week DB period.
Discussion
The ELECT study reported a reduced need for sc octreotide to control symptoms in patients with CS treated with lanreotide compared to placebo. However, the importance of this treatment effect in terms of patient-reported outcomes has not been quantified yet. In this analysis, we aimed to quantify the clinical benefit of lanreotide on patient-reported outcomes collected during the DB part of the ELECT trial. We first determined the most patientrelevant outcomes, calculated a clinically meaningful difference for each, and compared the numbers of patients who experienced a change from baseline larger than this threshold.
The current analysis highlights the important aspects of QoL extending beyond CS symptoms (ie, diarrhea and flushing). The first PC, which per definition captures the largest amount of information from the patient-reported dataset, was highly correlated to the C30-SS, indicating that aspects of QoL related to patient daily functioning, such as physical and emotional functioning, and nonspecific symptoms like pain and fatigue are clearly important to patients. These findings demonstrate that living with a symptomatic, inoperable, and ultimately fatal tumor is heavily affecting many facets of patients’ everyday lives. Importantly, the PCA clearly identified the burdens of diarrhea and flushing as other relevant and distinct aspects impacting patients’ lives. The analysis also showed an association between increased sc octreotide use and decreasing odds of responding on the C30-SS and BD, confirming the relevance of the primary endpoint in the ELECT clinical trial, which showed that lanreotide significantly reduced the percentage of days of sc octreotide use compared to placebo (absolute difference = –14.8%; 95% CI = –26.8 to –2.8).13 This analysis contributes to translating the treatment effect on the primary outcome as observed in the ELECT study into a functional outcome that may be more meaningful from the perspective of the patient.
The complex nature of evaluating QoL in NET patients has been investigated in the previous observational research, which has highlighted its importance in addition to symptoms of CS.15–17 A recent patient survey using the Patient-Reported Outcome Measure Information System (PROMIS) and Short Form-36 (SF-36) questionnaires to measure QoL in NET patients reported lower QoL compared to the general population on all subscales, which was primarily driven by patients with NET and CS.15 In further analysis of the data, a significant association was found between the number of flushing events and bowel movements and QoL, with an increased prevalence and severity of CS symptoms directly related to a reduction in QoL.16 In an additional global survey of 1928 NET patients, a large proportion of patients experienced diarrhea (48%) and flushing (37%), with many reporting the symptoms. Further, 92% of patients stated living with NETs required lifestyle modification daily (41% and 46%, respectively), including dietary and travel.17
The effects of somatostatin analogs on the symptoms of CS have been well established.18 The ELECT trial confirmed the efficacy of lanreotide in treating CS symptoms through the reduction of sc octreotide use compared to placebo.13 Previous post hoc analyses of the ELECT data have demonstrated that lanreotide patients were responsive with respect to their reported reduction in the symptoms of CS, and this was consistent across subgroups. In a post hoc analysis where patient-reported symptom data were combined to provide an average daily composite score, based on daily frequency and severity of symptoms, patients treated with lanreotide showed greater reductions in diarrhea or flushing compared with patients treated with placebo.19 Further subgroup analysis of ELECT found that the benefit of lanreotide compared to placebo, including greater CS symptom control, was consistent across treatment-naïve and octreotide-experienced patients, where lanreotide-treated subjects showed greater carcinoid symptom control in both samples.20 Results from the current analysis provide a deeper understanding of the response to lanreotide across multiple domains.
This evaluation of the patient-reported outcomes provides additional insight into the direct clinical benefits of lanreotide in patients with CS. However, with 42.9% of the baseline variation explained by the C30-SS, diarrhea burden, and flushing burden, the dataset is clearly multidimensional, which, from a clinical perspective, is not surprising. CS is characterized by an indolent course that may develop over many years, and the expression of symptoms experienced by individuals may be variable. Due to the heterogeneity of the clinical manifestations of CS, it was anticipated that no single measure would be able to broadly capture treatment benefit. It is not unexpected, therefore, that multifaceted QoL issues such as cognitive, physical, role or social functioning, as well as fatigue, insomnia, body image, appetite loss, and non-diarrhea GI symptoms, make up such a large part of a patient’s experience (Supplementary Annex 1, Figure S1A–C). Despite this, diarrhea and flushing symptoms were clearly expressed as the next most important PCs.
This analysis is not without limitations. First, it was conducted as a post hoc evaluation and describes trends that are descriptive in nature. The analysis was not statistically powered, and no statistical conclusions can be drawn from the data. To address this limitation, a PCA methodology was chosen to characterize the interrelationship of baseline patient-reported outcomes because it allows for objective, data-driven choices in the selection of relevant summary scores and composite variables. Second, the ability to construct anchor-based MCIDs for the patient-centered outcomes was limited. A consensus regarding the appropriate method to determine MCID is lacking. Additionally, in CS there is no established objective external criterion to distinguish responders from nonresponders on PRO patient-centered endpoints. Further, the ELECT trial did not include a global assessment of treatment efficacy (by either the subject or the investigator). Efforts were made to translate PRO and MCID scores into meaningful changes for the patients with CS, although further research is needed to establish the most useful concept of MCID such that it can alert the physician and the patient that treatment is effective and impacts a patient’s life or, alternatively, that a change in treatment is required. Third, a proportion of patients could not qualify as responders in this analysis. Of the total 115 patients who were randomized, 39 (34%) and 44 (38%) of patients had baseline BD and BF scores below the respective MCID thresholds. By definition, such patients cannot become responders in an MCID-defined responder analysis. It is possible that this lack of “responder potential” within the study population played a role in the lack of significance for the flushing burden endpoint. Composite response scores were explored to mitigate this situation. Additionally, the cumulative distribution function curves provide important information that contextualizes the results of the responder analysis. The curves clearly demonstrate a difference in the percentage of responders regardless of the chosen MCID in all three PCs when comparing lanreotide- and placebo-treated patients.
Conclusions
We used PCA to identify the most important patientreported outcomes among the patients included in the ELECT trial. The CS symptoms of diarrhea and flushing that have been previously reported as related to overall QoL in patients with NETs were identified as the most clinically relevant to patients. Treatment with lanreotide resulted in improved response rates for the C30-SS and BD scores after 12 weeks. Further, increased use of rescue sc octreotide was associated with lower odds of response to all three function response variables and overall composite score, which suggests that increased use of shortacting octreotide in the ELECT trial was associated with poor QoL and CS symptom control. These findings highlight that patient response to lanreotide treatment, not only with respect to symptoms of CS, improves functional and symptoms scores as indicated by the summary score of the QoL instrument.
Ethics Approval And Consent To Participate
ELECT (Clinicaltrials.gov: NCT00774930) began in May 2009; last-patient-last-visit for the double-blind phase occurred in May 2013. All procedures conformed to the Declaration of Helsinki, International Conference on Harmonisation Consolidated Guideline on Good Clinical Practice, and all national and local regulatory requirements.
Abbreviations
BD, Burden of diarrhea; BF, burden of flushing; C30-SS, C30 summary score; CI, confidence interval; CS, carcinoid syndrome; DB, double-blind; EORTC, European Organisation for Research and Treatment of Cancer; FDA, US Food and Drug Administration; GI, gastrointestinal; MCID, minimal clinically important difference; NET, neuroendocrine tumor;OR, odds ratio; PC, principal component; PCA, principal component analysis; PRO, patient-reported outcome; PROM, patient-reported outcome measure; QoL, quality of life; ROC, receiver operator characteristic; RR, Risk ratio; SC, subcutaneous; SEM, standard error of measurement.
Availability Of Data And Material
Where patient data can be anonymized, Ipsen will share all individual participant data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted to DataSharing@Ipsen.com and will be assessed by a scientific review board. Data are available beginning 6 months and ending 5 years after publication; after this time, only raw data may be available.
Acknowledgments
The authors thank Susan Pitman Lowenthal, MD, MPH for her contribution to the manuscript and Philip Sjostedt, BPharm, of The Medicine Group, LLC (New Hope, PA) for medical writing and editorial assistance, which was funded by Ipsen Biopharmaceuticals, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: The GPP3 Guidelines” and the International Committee of Medical Journal Editors’ “Uniform Requirements for Manuscripts Submitted to Biomedical Journals.”
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This post hoc analysis, as well as medical writing and editorial assistance, was sponsored by Ipsen Biopharmaceuticals, Inc.
Disclosure David Ray, Beloo Mirakhur, and Nilani Liyanage were paid employees of Ipsen Biopharmaceuticals, Inc. Luc Duchateau was financially supported by Ipsen to perform the analysis. Benedicte Lescrauwaet is an employee of Xintera Bvba. The authors report no other conflicts of interest in this work.
References
1. Rindi G, Capella C, Solcia E. Introduction to a revised clinicopathological classification of neuroendocrine tumors of the gastroenteropancreatic tract. Q J Nucl Med. 2000;44(1):13–21.
2. Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med. 1999;340 (11):858–868. doi:10.1056/NEJM199903183401107
3. Jensen R, Doherty G. Carcinoid tumors and the carcinoid syndrome. In: DeVita VT Jr., Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott Williams & Wilkins; 2001:1813–1833.
4. Soga J, Yakuwa Y, Osaka M. Carcinoid syndrome: a statistical evaluation of 748 reported cases. J Exp Clin Cancer Res. 1999;18(2):133– 141.
5. Vinik AI, Woltering EA, Warner RR, et al. NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39 (6):713–734. doi:10.1097/MPA.0b013e3181ebaffd
6. Thorson A, Biorck G, Bjorkman G, Waldenstrom J. Malignant carcinoid of the small intestine with metastases to the liver, valvular disease of the right side of the heart (pulmonary stenosis and tricuspid regurgitation without septal defects), peripheral vasomotor symptoms, bronchoconstriction, and an unusual type of cyanosis; a clinical and pathologic syndrome. Am Heart J. 1954;47(5):795–817. doi:10.1016/0002-8703(54)90152-0
7. Rohaizak M, Farndon JR. Use of octreotide and lanreotide in the treatment of symptomatic non-resectable carcinoid tumours. ANZ J Surg. 2002;72(9):635–638.
8. Fitzpatrick R, Davey C, Buxton MJ, Jones DR. Evaluating patientbased outcome measures for use in clinical trials. Health Technol Assess. 1998;2(14):i-iv, 1–74.
9. Food and Drug Administration (FDA). Guidance for industry - Patientreported outcome measures: use in medical product development to support labeling claims. 2009. Available from: http://www.fda.gov/down loads/Drugs/.../Guidances/UCM193282.pdf. Accessed July 14, 2016.
10. Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968–977. doi:10.10 16/S0140-6736(15)00817-X
11. Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of (177)Ludotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376 (2):125–135. doi:10.1056/NEJMoa1607427
12. Caplin ME, Pavel M, Cwikla JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371 (3):224–233. doi:10.1056/NEJMoa1316158
13. Vinik AI, Wolin EM, Liyanage N, Gomez-Panzani E, Fisher GA, ELECT Study Group. Evaluation of lanreotide depot/autogel efficacy and safety as a carcinoid syndrome treatment (ELECT): a randomized, double-blind, placebo-controlled trial. Endocr Pract. 2016;22(9):1068–1080. doi:10.4158/EP151172.OR
14. Giesinger JM, Kieffer JM, Fayers PM, et al. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol. 2016;69:79–88. doi:10.1016/j.jclinepi.2015.08.007
15. Beaumont JL, Cella D, Phan AT, Choi S, Liu Z, Yao JC. Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas. 2012;41(3):461–466. doi:10.1097/MPA.0b013e3182328045
16. Pearman TP, Beaumont JL, Cella D, Neary MP, Yao J. Health-related quality of life in patients with neuroendocrine tumors: an investigation of treatment type, disease status, and symptom burden. Support Care Cancer. 2016;24(9):3695–3703. doi:10.1007/s00520-016-3189-z
17. Singh S, Granberg D, Wolin E, et al. Patient-reported burden of a neuroendocrine tumor (NET) diagnosis: results from the first global survey of patients with NETs. J Glob Oncol. 2017;3(1):43–53. doi:10.1200/JGO.2015.002980
18. Kvols LK, Moertel CG, O’Connell MJ, Schutt AJ, Rubin J, Hahn RG. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986;315(11):663– 666. doi:10.1056/NEJM198609113151102
19. Fisher GA Jr., Wolin EM, Liyanage N, et al. Patient-reported symptom control of diarrhea and flushing in patients with neuroendocrine tumors treated with lanreotide depot/autogel: results from a randomized, placebo-controlled, double-blind and 32-week open-label study. Oncologist. 2018;23(1):16–24. doi:10.1634/theoncologist.
20 17-0284 20. Fisher GA, Pommier RF, Wolin EM, et al. Lanreotide depot (LAN) for symptomatic control of carcinoid syndrome (CS) in neuroendocrine tumor (NET) patients previously responsive to octreotide (OCT): subanalysis of patient-reported symptoms from the phase III elect study. J Clin Oncol. 2017;35(15_suppl):4088. doi:10.1200/ JCO.2017.35.15_suppl.4088

